Research Overview
In the Interdisciplinary NanoBioSciences Lab, we work largely at the interface of bioengineering and the biological sciences. The complexity of disease necessitates creative, multi-faceted solutions that bioengineers have the potential to offer. At the same time, biological models that faithfully recapitulate human disease and increase our understanding of molecular pathophysiology will improve the design of treatments. Ultimately, our scientific goals are to: 1.) better understand biomaterial-biological system interactions to improve the performance of biomaterials in humans, 2.) discover new molecular targets ideal for biomaterials-based therapies, and 3) identify cellular and molecular processes that impact drug delivery efficiency and resistance to therapy.
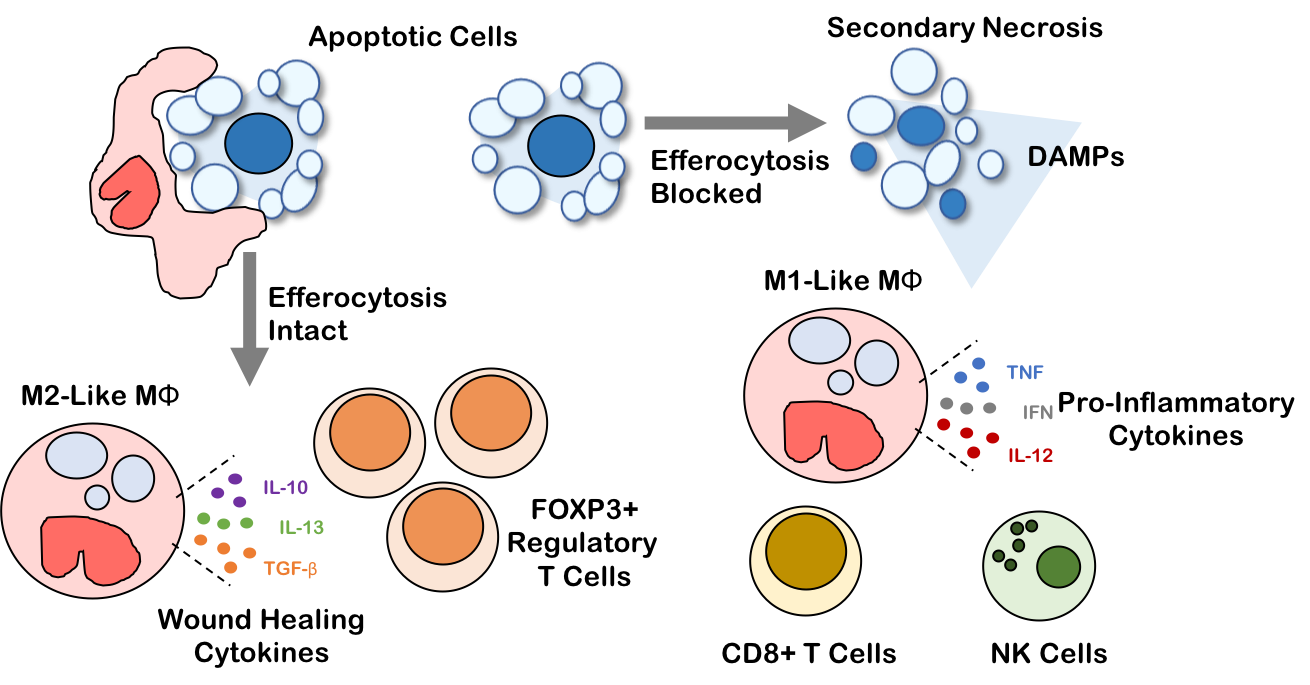
Efferocytosis in the tumor microenvironment
Apoptotic cells are frequently observed in cancers, and increase to an even greater extent upon cancer treatment. A mechanism exists to swiftly remove apoptotic cells from both untransformed and neoplastic tissue prior to their secondary necrosis, in order to prevent inflammation-mediated tissue damage. This mechanism to remove apoptotic cells is efferocytosis, a process by which apoptotic cells are precisely identified and engulfed by a neighboring phagocytic cell (macrophages in the tumor microenvironment). Efferocytosis by tumor macrophages has an immunosuppressive, pro-tolerogenic impact on the tumor microenvironment, perhaps driving malignant tumor progression. We are exploring new molecular targets to block efferocytosis in the tumor microenvironment in an effort to boost anti-tumor immunity of cancer immunotherapies such as checkpoint inhibitors.
Relevant Publications
Werfel T. A. Assessment of the Immune Response to Tumor Cell Apoptosis and Efferocytosis. Apoptosis and Cancer (Methods in Molecular Biology). 2022. 45-55. Full Text
Shofolawe-Bakare O. T., Stokes L. D., Hossain M., Smith A. E., Werfel T. A. Immunostimulatory Biomaterials to Boost Tumor Immunogenicity. Biomaterials Science. 2020, DOI: 10.1039/D0BM01183E.*2021 Emerging Investigators Special Edition. Full Text
Werfel T. A., Elion D. L., Rahman B., Hicks D. J., Sanchez V. M., Gonzalez Ericsson P., Nixon M. J., James J., Balko J. M., Scherle P. A., Koblish K., Cook R. S. Treatment-induced tumor cell apoptosis and secondary necrosis drive tumor progression in the residual tumor microenvironment through MerTK and IDO-1. Cancer Research. 2019, 10.1158/0008-5472.CAN-18-1106. Full Text
Werfel T. A., Cook R. S. Efferocytosis in the tumor microenvironment. Seminars in Immunopathology. 2018, https://doi.org/10.1007/s00281-018-0698-5. Full Text
Funding
NIH P20 GM130460-01A1 7937
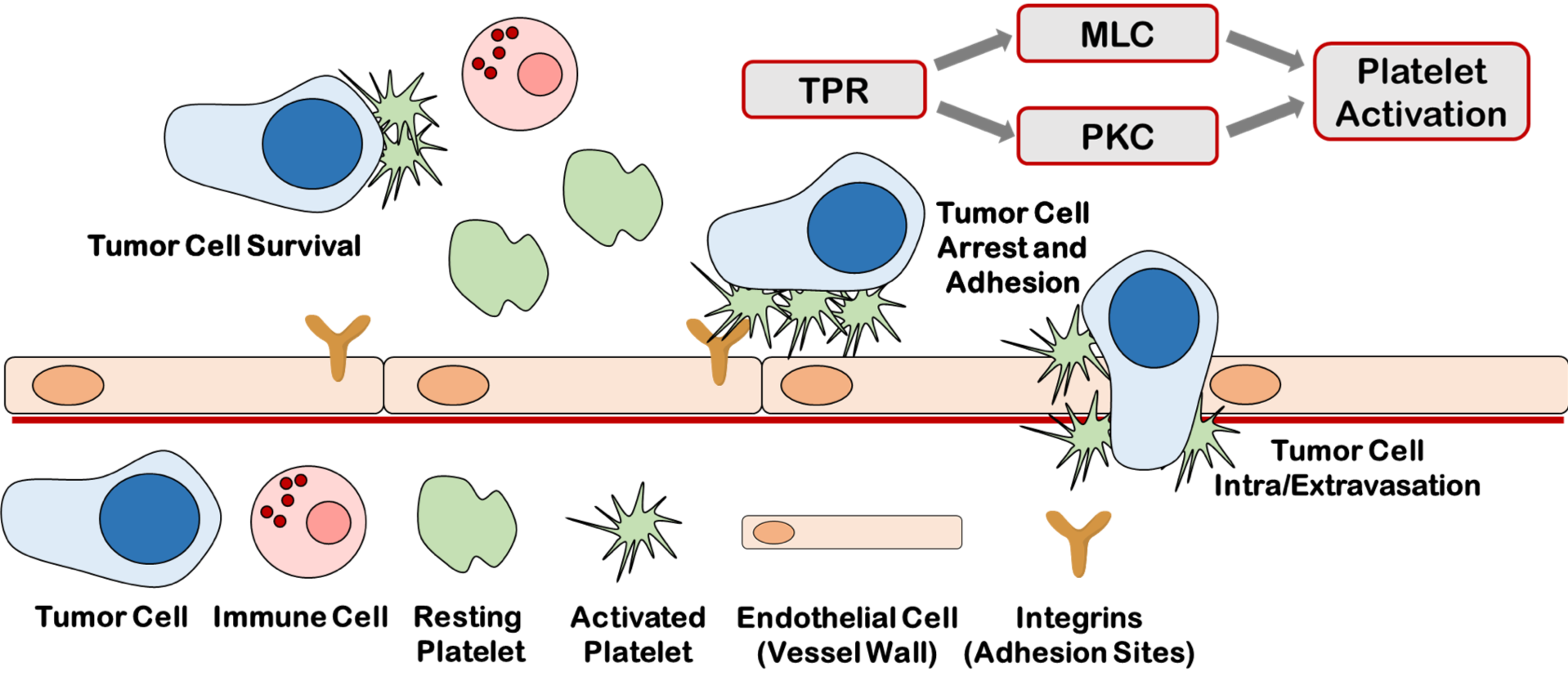
Platelet-mediated cancer metastasis
Once a breast cancer is diagnosed as metastatic, the cancer becomes a disease that is managed, but no longer curable. Although cytotoxic and molecularly-targeted therapies that target cancer cell survival and/or proliferation exist, there are currently few approaches to neutralize the metastatic spread of tumor cells. Agents that block, slow, or interfere with the metastatic process would address an enormous unmet medical need and reconcile the debilitating emotional toll on people suffering with late-stage cancer. We have developed the working hypothesis that platelet-activating signaling promotes breast cancer metastasis by increasing platelet-tumor cell aggregation, arrest, adhesion, and extravasation at metastatic sites. By investigating the role of platelets in breast cancer metastasis, our goal is to provide mechanistic insight into the interaction of platelets and tumor cells that assists tumor cell dissemination and translate new molecularly-targeted therapies that target the platelet-tumor cell interaction in breast cancer metastasis.
Relevant Publications
Toragall V. B., Hale E. J., Hulugalla K. R., Werfel T. A. Microscopy and Plate Reader-based Methods for Monitoring the Interaction of Platelets and Tumor Cells in vitro. Bio-protocol. 2023. 13(20). Full Text
Werfel T. A., Hicks D. J., Rahman B., Bindeman W., Duvernay M., Maeng J., Hamm H., Lavieri R., Morrison Joly M., Pulley J., Elion D., Brantley-Sieders D., Cook R. S. Repurposing of a Thromboxane Receptor Inhibitor Based on a Novel Role in Metastasis Identified by Phenome Wide Association Study. Molecular Cancer Therapeutics. 2020, 19(12), 2454-2464. Full Text
Funding
American Cancer Society RSG-21-114-01-MM
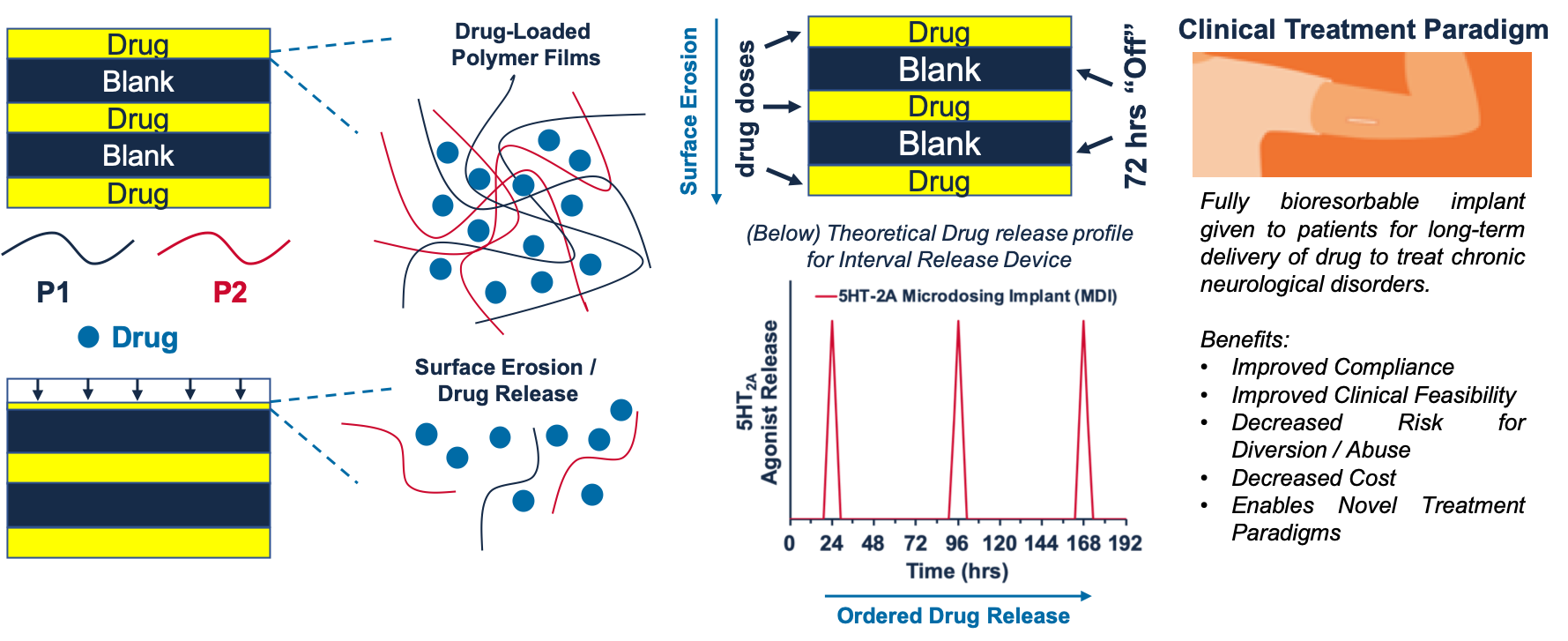
Interval drug delivery to treat chronic neurological disorders
There is an urgent unmet medical need to provide long-lasting symptom relief for patients suffering from a variety of chronic neurological disorders including major depressive disorder and addiction. Existing psycho- and pharmaco-therapies have limited impact and often require lengthy and burdensome treatment protocols with frequent follow-up. We are developing biomaterials-based approaches to ensure long-term interval dosing of patients to: 1) ensure compliance and safety of existing pharmacotherapies with complex dosing regimens, and 2) enable novel paradigms for pharmacotherapy of chronic neurological disorders.
Relevant Publications
Brewster P. R., Ishraq Bari S. M., Walker G. M., Werfel T. A. Current and Future Directions of Drug Delivery for the Treatment of Mental Illnesses. Advanced Drug Delivery Reviews. 2023. 114824. Full Text
Hossain M., Sulochana S. P., Heath K. E., Ishraq Bari S. M., Brewster P., Barnes J., Munivar A., Walker G. M., Puleo D. A., Werfel T. A. Interval Delivery of 5HT2A Agonists Using Multilayered Polymer Films. Journal of Biomedical Materials Research Part A. 2023. 10.1002/jbm.a.37497. Full Text
Funding
NIH P30 GM122733-03A1 5347
NIH R21 EB031454-01A1
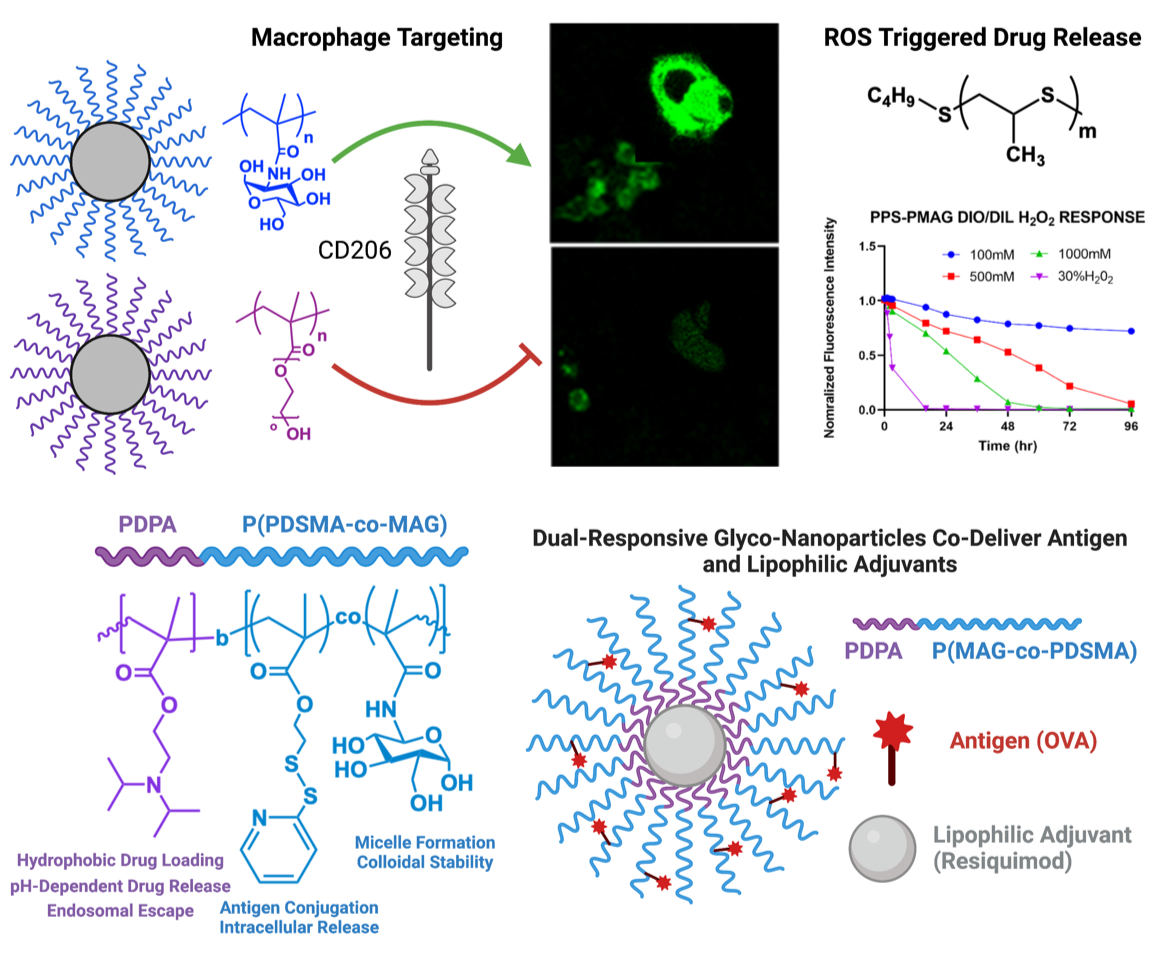
“Smart” glycopolymer-based nanomaterials for targeted drug delivery
We have broad expertise in the design of “smart”, or environmentally-responsive, polymers for drug and gene delivery. We employ pH, redox, and other environmental triggers to release drug in response to pathological cues. Our lab is actively developing glycopolymers for targeted delivery to the innate immune system and “catch and release” polymer systems for efficient nucleic acid delivery.
Relevant Publications
Mohammad S. A., Toragall V. B., Fortenberry A. W., Shofolawe-Bakare O. T., Sulochana S., Heath K. E., Owolabi I., Tassin G., Flynt A. S., Smith A. E., Werfel T. A. Postpolymerization Modification of Poly(2-vinyl-4,4-dimethyl azlactone) as a Versatile Strategy for Drug Conjugation and Stimuli-Responsive Release. Biomacromolecules. 2024. 25, 4, 2621-2634. Full Text
De Mel J., Hossain M., Shofolawe-Bakare O. T., Mohammad S. K., Rasmussen E., Milloy K., Shields M., Roth E. W., Arora K., Cueto R., Tang S., Wilson J. T., Smith A. E., Werfel T. A. Dual-Responsive Glycopolymers for Intracellular Codelivery of Antigen and Lipophilic Adjuvants. Molecular Pharmaceutics. 2022, 19(12), 4705-4716. Full Text
Fortenberry A. W., Mohammad S. A., Werfel T. A., Smith A. E. Comparative Investigation of the Hydrolysis of Charge-Shifting Polymers Derived from an Azlactone-Based Polymer. Macromolecular Rapid Communications. 2022. 2200420. Full Text
Shofolawe-Bakare O. T., de Mel J. U., Mishra S. K., Hossain M., Hamadani C. M., Pride M. C., Dasanayake G. S., Monroe W., Roth E. W., Tanner E. E. L., Doerksen R. J., Smith A. E., Werfel T. A. ROS-Responsive Glycopolymeric Nanoparticles for Enhanced Drug Delivery to Macrophages. Macromolecular Bioscience. 2022. 2200281. Full Text
Funding
NSF CAREER 2141666